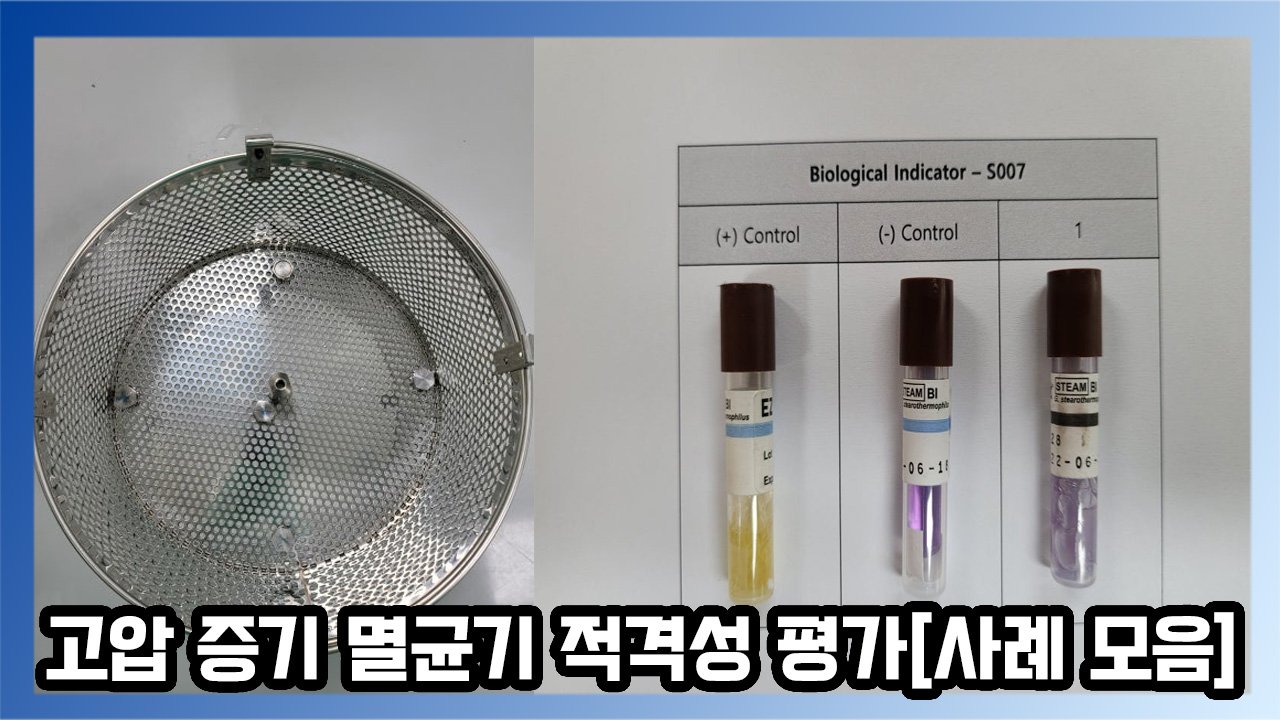
What is an Autoclave (High-Pressure Steam Sterilizer)?
An autoclave is a sterilizer that uses high-pressure, high-temperature steam to sterilize equipment. It is commonly used in sensitive environments such as laboratories, where bacteria, pathogens, and microorganisms must be controlled, as well as in the medical field to sterilize surgical instruments and dressings to prevent infection. Compared to other sterilization methods, such as dry heat sterilizers, autoclaves have the advantage of shorter processing times.
Qualification Assessment Items
Since autoclaves operate at high temperatures, many of the qualification assessment items are related to temperature.
1. Heat Distribution Verification






Temperature data loggers are installed at various positions in the basket inside the autoclave to verify that the internal temperature is evenly distributed.
2. Heat Penetration Verification






This assessment is performed by placing actual items to be sterilized in the autoclave basket, as would be done in the client’s real sterilization process. Temperature data loggers are placed between the items to ensure that heat is evenly distributed and that the items receive adequate heat during the process.
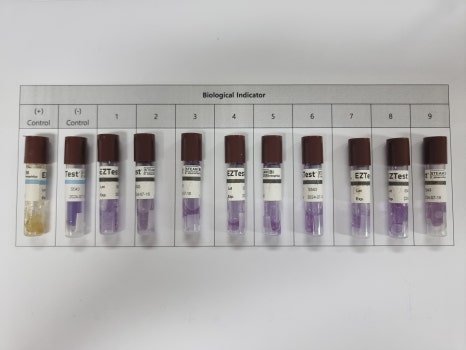
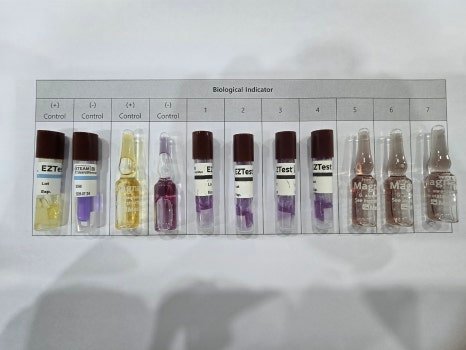
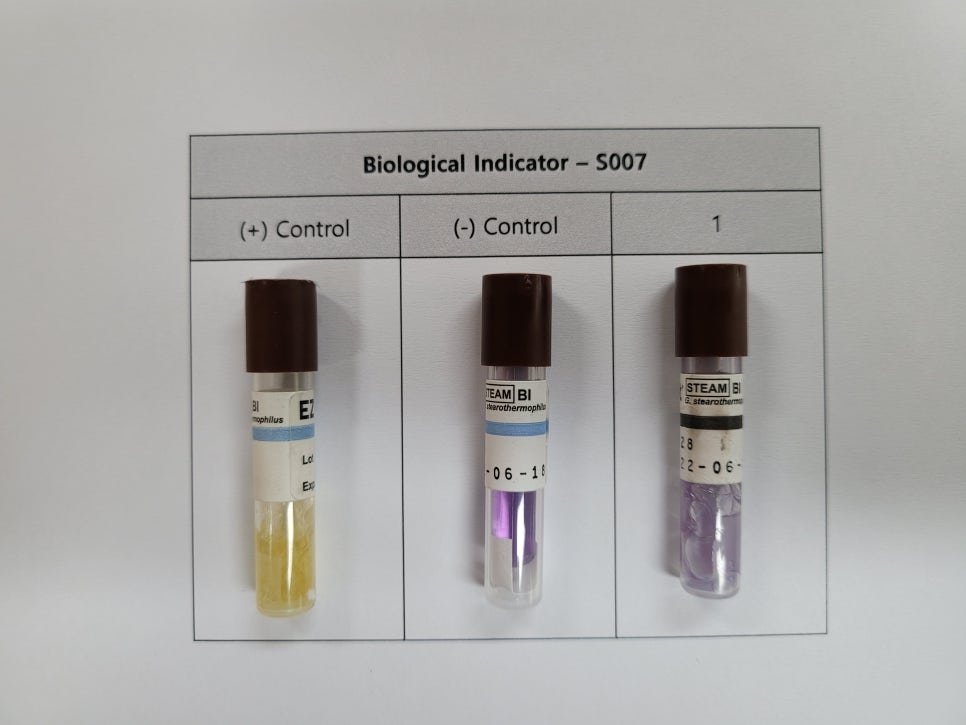
3. Biological Sterilization Verification


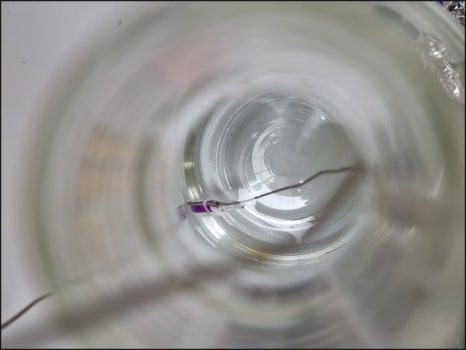


This item evaluates the sterilization effectiveness of the autoclave. It is often performed together with heat penetration verification. Biological Indicators (BIs) are placed among the items being sterilized, along with temperature data loggers, to confirm that sterilization is effective.
Note:
A BI (Biological Indicator) is a test device containing a specified number of highly resistant microorganisms. It is used to assess whether the sterilization process is effective. For autoclaves, heat- and moisture-resistant microorganisms are used.
4. Pressure Verification

Often performed together with heat distribution verification, this assessment uses pressure sensors installed inside the basket to confirm that the autoclave maintains proper internal pressure during operation.
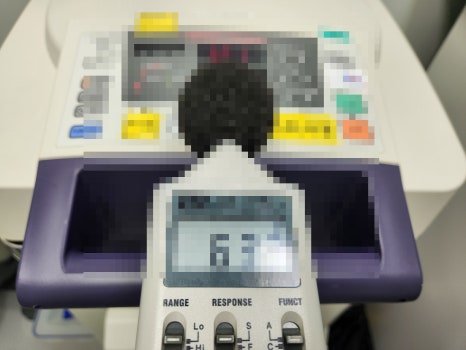
5. Noise Verification
A decibel meter is used to check whether the noise level during operation is within the normal range, helping to identify any potential malfunctions.
Case Collection
1. Pangyo Laboratory Case


Heat distribution verification



Heat penetration and biological sterilization verification performed together
(If there are various ways to arrange items, each arrangement is tested.)


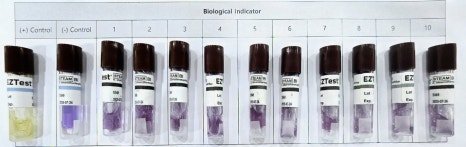
After the assessment, all data is converted into numerical values and documented for record-keeping.
2. Hwaseong Laboratory Case
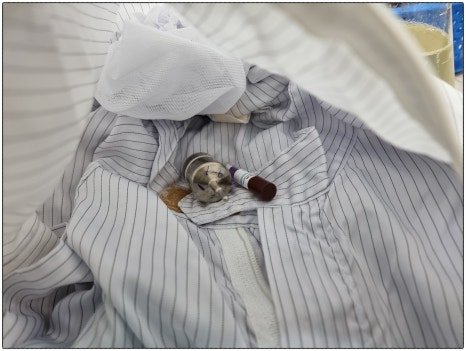



Heat penetration and biological sterilization verification were performed, omitting heat distribution verification.


3. Gangnam Company Case

Four assessment items: noise verification, heat distribution verification, heat penetration verification, and biological sterilization verification.









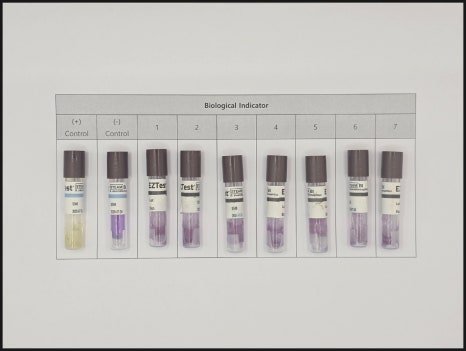
4. Ansan Factory Laboratory Case



Heat distribution verification followed by heat penetration and biological sterilization verification.


Note: Data has been anonymized to protect company information.
5. Ansan Factory Media Disposal Room
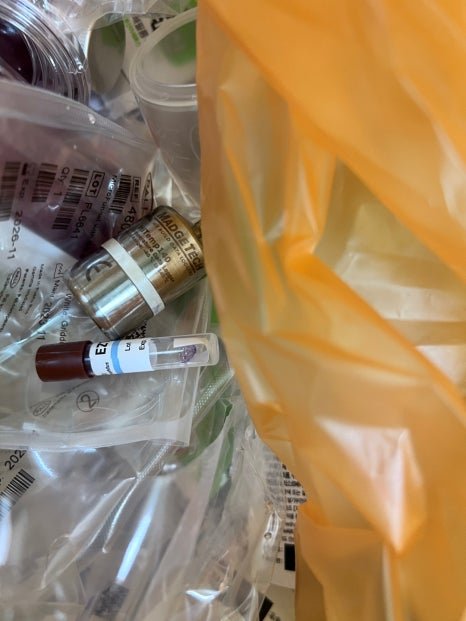
Qualification assessment of an autoclave used to prevent the release of microorganisms from the factory’s media disposal room.

Heat distribution, heat penetration, and biological sterilization verification were performed.


Note: Data has been anonymized to protect customer information.
6. Hangnam Research Institute

Heat penetration and biological sterilization verification were performed.
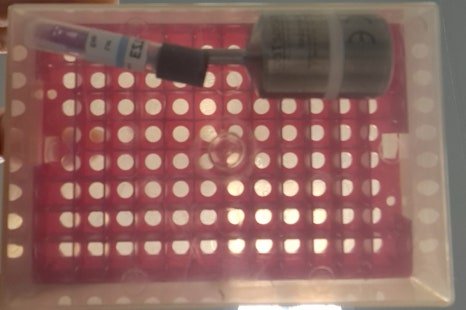





7. Pangyo Laboratory Case 2 (Different Company)


Heat distribution, heat penetration, and biological sterilization verification were performed.


8. Pangyo Research Institute Re-Qualification Assessment

Regular re-qualification is required to maintain compliance, especially after part replacement, relocation, modification, or malfunction.



Heat distribution, heat penetration, and biological sterilization verification were performed.

9. Seongnam Company Re-Qualification Assessment
Regular re-qualification is required as above.
Load condition heat distribution, heat penetration, and biological sterilization verification were performed.


10. Incheon Research Institute

Only heat distribution verification was performed.
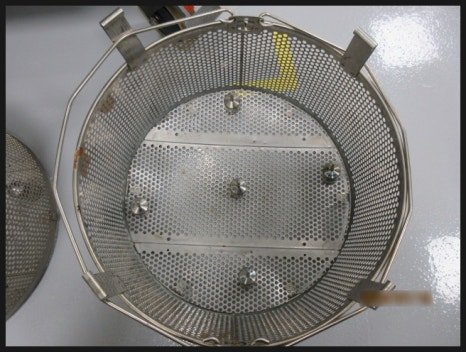

11. Seongnam Pangyo Company
Re-qualification assessment for a previously qualified company.
Heat distribution, heat penetration, and biological sterilization verification were performed.







12. Pangyo Laboratory

Re-qualification assessment for a previously qualified site.
Heat distribution, heat penetration, and biological sterilization verification were performed.




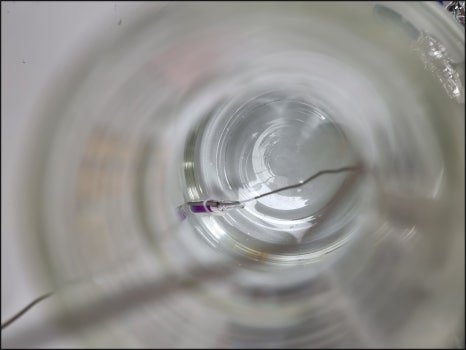



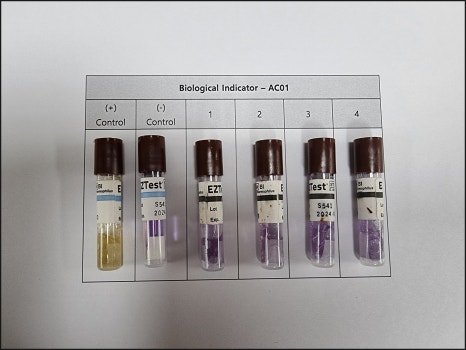
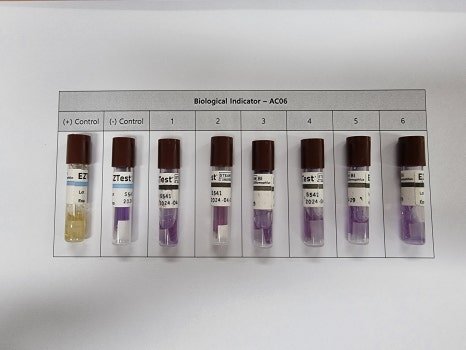
All measured data is recorded and documented.
13. Seoul Gangnam Pharmaceutical Company
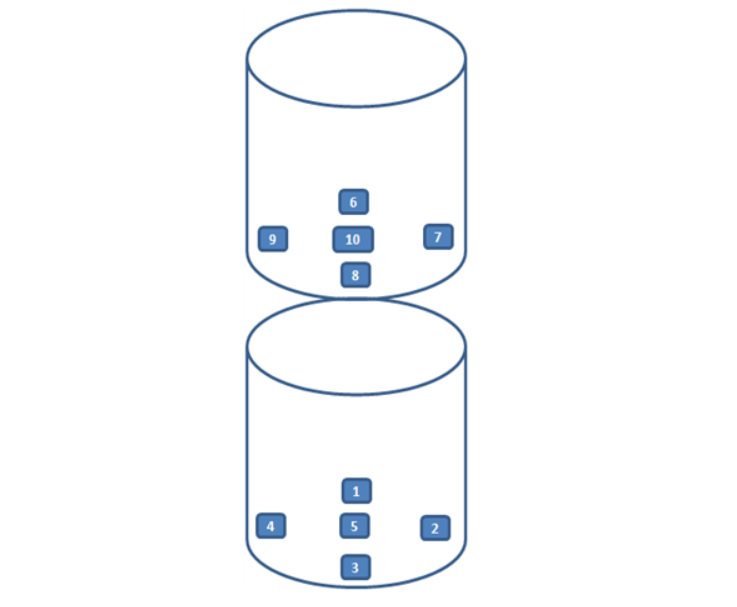
Re-qualification assessment for a previously qualified company.
Noise verification, heat distribution, heat penetration, and biological sterilization verification were performed.



All measurement data was documented.


14. Pangyo Research Institute
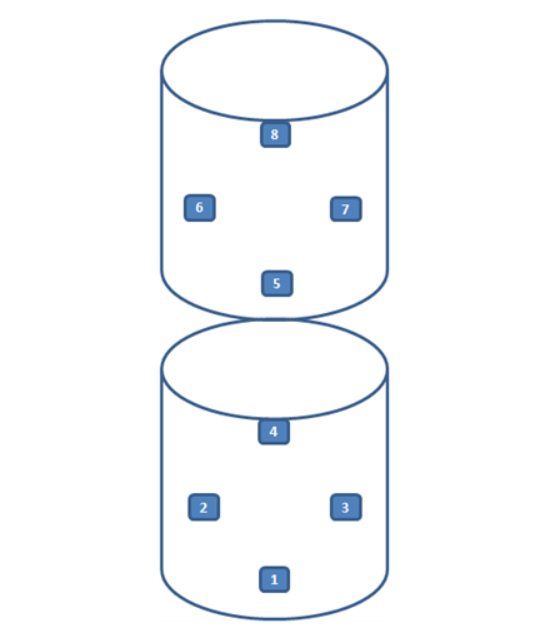
Re-qualification assessment for a previously qualified company.


Heat distribution, heat penetration, and biological sterilization verification were performed.








All measured data was recorded and documented.
15. Bucheon Factory

Re-qualification assessment for a previously qualified company.

Pressure verification and heat distribution verification were performed together, followed by heat penetration and biological sterilization verification.




Contact Us
If you have any questions or need a solution, please let us know your required specifications and intended use. You can leave your inquiry in the quotation request section at the bottom of this page, and we will be happy to assist you.
CAS Scale Korea is always committed to providing you with useful information.











No Comments